Johnson & Johnson Offers $2.5 Billion to Settle DePuy Hip Lawsuits
Attorneys for Johnson & Johnson said that the medical device manufacturing giant will pay approximately $2.5 billion to settle some 8,000 lawsuits brought by plaintiffs, who claim they have suffered serious complications from defective DePuy ASR hip implants.
The proposed settlement is less than what Bloomberg News had speculated in late November when it reported that J&J was prepared to pay out a record $4 billion to settle those same cases.
The agreement, which was announced in the Federal District Court in Toledo, specifies that $2 billion will be set aside for basic awards. An additional $475 million has been reserved for people who suffered more extensive injuries. J&J also announced that medical costs for those patients would be paid under a separate $3 billion dollar fund.
Multimillion Dollar ASR Hip Implant Verdict Upheld by Judge
If Johnson & Johnson (J&J) attorneys were expecting a Los Angeles judge to overturn a historic $8.3 million jury verdict for the plaintiff in the first DePuy ASR hip case to go to trial, they went home disappointed.
In March, a jury found that J&J’s DePuy Orthopaedics subsidiary had manufactured a defective metal-on-metal hip replacement device and failed to adequately warn patients about the risks of the hip implant. The plaintiff in the trial alleged that defects with the design of DePuy’s ASR hip had caused him to suffer permanent injuries.
The jury agreed and awarded him $8.5 million in damages. Attorneys representing DePuy asked the judge to overturn the verdict and grant a new trial, claiming that there was not enough evidence presented at the trial to justify the verdict.
The judge disagreed with that assessment and let the verdict stand. He said that based on the evidence, it was not unreasonable for a jury to conclude that the ASR hip device was defective and that DePuy had not adequately warned patients about potential complications associated with the implant.
There has only been one other case concerning the DePuy ASR hip that has gone to trial so far. An Illinois jury found for DePuy in that case. Currently, there are more than 10,000 cases pending in federal and state courts alleging similar complications with the metal-on-metal hip implant.
In 2010, DePuy issued a voluntary recall of its ASR product line. At the time, the company said the recall was not an admission that the devices were defective, but reflected a marketing decision. However internal memos made public during the trial indicated that DePuy knew there were problems with the ASR devices before the recall.
Recently, DePuy decided to phase out all of its controversial metal-on-metal hip products.
Do you believe you have suffered an injury due to a defective metal-on-metal hip device? Contact the experienced metal-on-metal hip implant failure attorneys at the Law Offices of Reneé J. Nordstrand. Our dedicated legal team is committed to seeking maximum compensation for our clients.
You can call the Law Office of Renee J. Nordstrand at our Santa Barbara office at (805) 962-2022 or our Encino office at (818) 981-3530 to discuss your legal rights.
J&J Hip Trial Testimony Alleges Company Knew of Defects Years before Recall
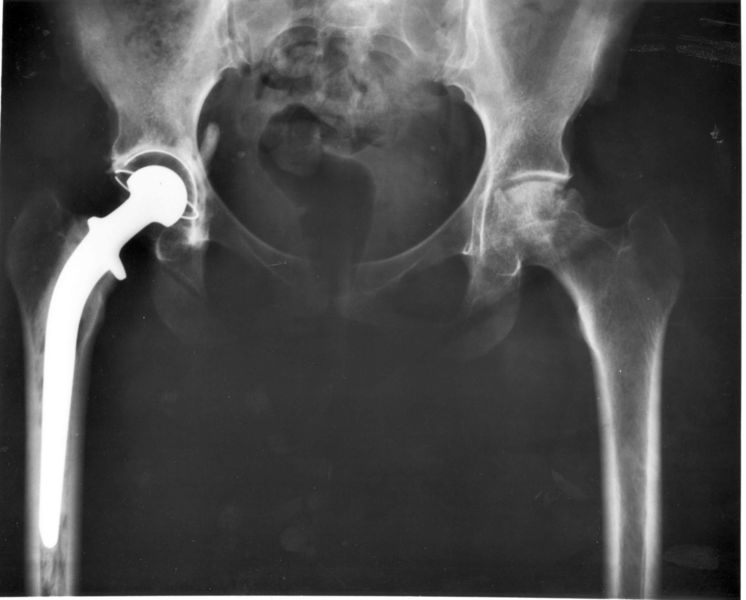
The J&J ASR hip replacement defect attorneys at the Law Offices of Reneé J. Nordstrand have been following the first Johnson & Johnson hip device trial that is currently being conducted in Los Angeles. We have learned that J&J may have known about the defects for years, but did nothing to address them.
A J&J DePuy unit bioengineer testified via videotape that he spent three years studying a redesign of the ASR hips to address the known high failure rate of the devices. However, the project (dubbed Project Alpha) went nowhere and no changes to the defective device were made. The project was terminated in 2008. J&J recalled the ASR hip implants in 2010.
Bloomberg News reports that the attorney for the plaintiff seeking damages against J&J pointed to an email the engineer sent to his superiors in April, 2008. In it he wrote, “a small improvement to geometry could represent a large improvement for many patients.” He then wrote that by simply removing a groove, wear on the cup of the device “is approximately threefold less than the original design with the groove.”
That same day he wrote an email to his superiors complaining that he was receiving “absolutely no direction” from them concerning his findings.
The plaintiff’s attorney claims his client was sickened by metal ions that had been released into his bloodstream due to the defective design of the ASR metal-on-metal hip replacement. Other patients have complained of pain, joint dislocations, bone fractures, and infections. Currently, there are some 10,000 lawsuits that have been filed concerning the recalled hip implants.
The Law Offices of Reneé J. Nordstrand will be keeping a close eye on the outcome of this bellwether trial. If you believe you’ve suffered an injury due to a defective J&J ASR metal-on-metal hip replacement, call our Santa Barbara office at (805) 962-2022 or our Encino office at (818) 981-3530. We can also be contacted online. Arrange for your free consultation today.
First J&J Hip Implant Trial Begins in Los Angeles
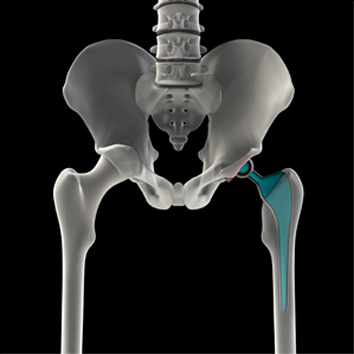
Opening arguments have been heard in the first Johnson & Johnson metal-on-metal ASR hip implant case to go to trial. The ASR hips, which were manufactured by J&J’s DePuy unit, were recalled in 2010 when it was found that the devices were failing at higher-than expected rates.
The ASR hip recall attorneys at the Law Office of Renée J. Nordstrand have been following the developments in defective metal-on-metal hips and have found that failures have led to loosening of the hip implant, pain, swelling, joint weakness, tumors, heavy metal poisoning and soft tissue damage around the joint in patients who were implanted with the devices.
According to Reuters, the plaintiff’s attorney claims that J&J knew of the product’s defects in 2004 before it started selling the ASR device — but decided to market it anyway. His 66-year-old client contends that the hip implant made him ill when it released metal ions into his blood, which elevated the levels of cobalt and chromium in his system.
The plaintiff’s attorney claimed that DePuy failed to warn patients and doctors about the defects in the all-metal ASR hip implants. “Doctors relied 100 percent on DePuy and patients relied 100 percent on doctors and information was kept from them,” he told the jury.
A J&J attorney countered that the evidence would show that the man had many health issues before the implant; and regardless of that, the level of metal in his blood was not high enough to make him ill. The attorney stated that DePuy is a “good and conscientious company.”
There are currently over 10,000 lawsuits that have been filed against J&J over its metal-on-metal hip replacements.
If you have been injured as a result of DePuy implant failure, you have the right to hold them liable for losses and damages you suffered as a result of their negligence. To learn more about your legal rights and ongoing litigation, contact The Law Office of Renée J. Nordstrand. Call our Santa Barbara office at (805) 962-2022 or our Encino office at (818) 981-3530. We can also be contacted online. Your consultation will be free and confidential.
DePuy Pinnacle Hip Implant Lawsuits Piling Up
DePuy Orthopaedics, a subsidiary of health care giant Johnson & Johnson, has been facing its fair share of problems over its defective ASR XL Acetabular System total hip replacement and it ASR Hip resurfacing system. Now, Drugwatch .com has revealed that as of November of this year, DePuy is facing nearly 2,700 lawsuits over its Pinnacle Hip Implants.
Unlike the ASR and ASR XL hip implant systems, the Pinnacle implant has yet to be recalled by Johnson & Johnson. DePuy currently is facing some 6,200 hip implant lawsuits for injuries from recalled ASR devices.
The burgeoning number of Pinnacle lawsuits, which have been filed in federal court, may be consolidated into a multidistrict litigation (MDL). An MDL consolidates cases that are similar in claims and scope to streamline the discovery phase. The first trial in the Pinnacle MDL is scheduled for May 2014.
Many of the lawsuits filed over the Pinnacle hip implants claim that the devices are defective and can cause serious adverse complications. Litigants claim that the metal-on-metal components rub against each other, which make the implants prone to early failure. This can lead to painful and potentially dangerous revision surgeries. There are also concerns about metallosis, a blood poisoning caused by microscopic bits of chromium and cobalt being released into the blood stream.
If you’ve been the victim of a Pinnacle hip implant failure that requires corrective surgery or have suffered adverse effects due to a defective DePuy hip implant device, a California hip implant failure lawyer at the Law Offices of Renée J. Nordstrand can help you hold the company liable for the losses and damages you’ve experienced. We are dedicated to obtaining maximum compensation for our clients
Contact our Southern California Offices in Encino (818) 981-3530 if you have a question about your legal rights. Your consultation is free.
The Lasting Effects of the DePuy Hip Implant Recall
DePuy recalled a hip replacement system known as the ASR XL Acetabluar System in 2010. Many patients who received this implant have had problems with the implant, which requires replacement or revision surgery. They have suffered pain, lost wages, medical expenses and inconvenience.
The DePuy ASR XL Acetabluar System was marketed in the United States in 2005 and placed in thousands of patients. Since 2008, the U.S. Food and Drug Administration (FDA) received hundreds of claims of problems about this implant. In March 2010 Johnson & Johnson, the parent company of DePuy, admitted that the ASR XL Acetabluar System had a much higher than expected failure rate and in August 2010, recalled the product. As a result of the defect, many patients must have the ASR XL Acetabluar System removed from their body and replaced. Patients may be left with significant long-term health problems as a result of the defective product.
As consumers, we trust and expect that the products we purchase and use are safe and free of any design flaw or manufacturing defect that could cause injury, illness, or death. This is especially the case with medical devices and implant systems. Unfortunately, due to various forms of negligence and oversight, defective and poorly designed products often make their way onto the market, causing consumers to suffer harm.
At the Law Offices of Renee J. Nordstrand, our Santa Barbara defective product attorneys have the legal skills and resources to hold negligent manufacturers legally responsible for consumer injury or wrongful death. If you or someone you care about has experienced DePuy hip implant failure, our lawyers can help you obtain compensation for medical expenses, lost wages, and other damages. For a free consultation, call (805) 962-2022.
Metal Corrosion of Femoral Stems in DePuy Hip Implants and Other Concerns
In a peer reviewed article, copyrighted in 1998 by The Journal of Bone and Joint Surgery, incorporated and funded by among others, Zimmer and DePuy, it was apparent that DePuy and Zimmer were aware that there were increased concentrations of metal in association with metal implants. Corrosion of orthopaedic implants was a serious clinical concern. Although the freely corroding implant materials used in the past had been replaced with modern corrosion-resistant superalloys, deleterious corrosion processes have been observed in certain clinical settings.
According to the New York Times, many artificial joints, including the products created by Zimmer and DePuy, do not have to be tested in patients or cleared by the U.S. Food and Drug Administration (FDA) in order for them to be placed on the market. With baby boomers making up a large population and slowly entering into the later years of their lives, many individuals are facing hip replacement surgery, which makes it more important than ever that hip implant systems are properly designed for efficiency and quality.
Hip implant failure presents many challenges for a person. Not only does a patient have to undergo corrective surgery to replace the implant, he or she must cope with paying medical bills, pain and suffering, physical therapy, and other challenges.
A hip replacement failure attorney in Santa Barbara at the Law Offices of Renee J. Nordstrand can inform patients who have experienced DePuy and Zimmer hip implant failure of their legal rights and options. Our lawyers have years of experience handling serious personal injury and product liability cases. To learn more about how we can help you obtain the compensation you deserve, call (805) 962-2022 for a free consultation.
DePuy ASR Hip Implant Patients Entitled to Immediate Reimbursement for Recall-related Expenses
The month of August in 2010 brought bad news for patients who had hip replacement surgery and received a DePuy Orthopaedics hip implant system. After receiving new data from the National Joint Registry (NJR) of England and Wales revealing that the five year revision rate for the ASR Hip Resurfacing System is about 12 percent and about 13 percent for the ASR XL Acetabular System, DePuy announced a recall of the defective hip implant systems.
Once DePuy receives confirmation that a patient has received an ASR hip implant, recall-related expenses can be documented and submitted for reimbursement. DePuy will address medical costs directly associated with the recall of the ASR Hip System. These costs relate to recall-related patient out-of-pocket expenses, such as co-pays, deductable expenses, lost wages and travel costs, related to revision surgeries. DePuy will cover expenses for testing and treatment. Although this seems simple enough, it is important for patients to ensure that their rights are protected during the process of obtaining full and just compensation from DePuy.
At the Law Offices of Renee J. Nordstrand, our dedicated Santa Barbara DePuy hip implant failure lawyers are dedicated to obtaining maximum compensation for each patient harmed by DePuy’s negligent hip implant testing and manufacturing. If you have suffered as the result of the DePuy implant in California, contact us today for a free consultation. Call (805) 962-2022.
DePuy Orthopaedics ASR Hip Implant Failure Statistics Prompts Recall
One of the most common ailments, especially for older adults, is arthritis, and one of the most commonly replaced joints is the hip. According to the New England Journal of Medicine, about 750,000 patients with advanced painful arthritis have a total hip replacement annually in the U.S. These patients trust that the surgery will improve their quality of life and restore mobility. Unfortunately, this is not the case with DePuy ASR hip implants.
DePuy Orthopaedics, Inc., a subsidiary of Johnson & Johnson, introduced the ASR XL Acetabular hip implant system in the U.S. in 2005. It sported an innovative metal-on-metal design, as opposed to the traditional metal ball inserted into a plastic cup of its predecessors. The ASR was constructed, not by developing a whole new product, but by retrofitting a metal alloy cup from the ASR Hip Resurfacing System onto a standard hip implant. DePuy did not conduct clinical trials for the ASR.
In 2010, after the DePuy artificial hip system had already been implanted in about 100,000 Americans, the National Joint Registry (NJR) of England and Wales released data showing a high rate of replacement, or revision, for the ASR. The data revealed a five-year revision rate of an estimated 13 percent for the ASR XL Acetabular System and 12 percent for the ASR Hip Resurfacing System, although the later was not used in the U.S. Additional reports concluded that 21 percent of DePuy ASR hip systems had to be replaced by four years. This jumps to 49 percent by six years. With this information, DePuy recalled the ASR XL artificial hip implants on August 24, 2010.
Hip replacement surgery is not a simple process; it is an invasive operation which requires months of recovery and rehabilitation. If the hip implant fails, the patient’s subjected to a hip revision surgery. Even if the hip implant does not completely fail, it could cause serious complications such as heavy metal poisoning and tumors around the implant. In any case, it is likely that the patient would experience increased pain and discomfort.
At the Law Offices of Renee J. Nordstrand, our dedicated Santa Barbara DePuy hip implant failure lawyers are dedicated to obtaining maximum compensation for each patient harmed by DePuy’s negligent hip implant testing and manufacturing. If you have suffered as the result of the DePuy implant in California, contact us today free consultation. Call (805) 962-2022.